Several cytokines, including IL-4, IL-13, and IL-31, contribute to type 2
inflammation and to the clinical disease features of atopic dermatitis that
may contribute to eczematous lesions, including2-9:
Barrier dysfunction
Increased risk of
skin infection
Intense itch
Click on the cytokine to see the role of type 2 inflammation in the disease process
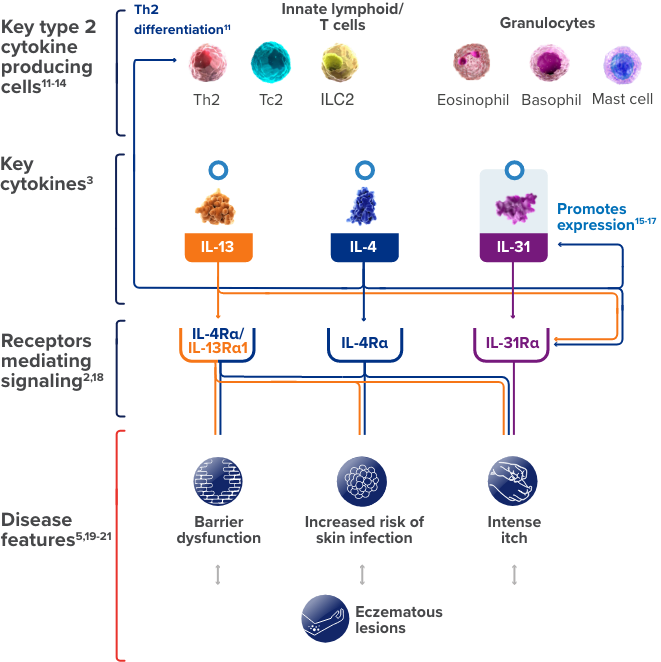
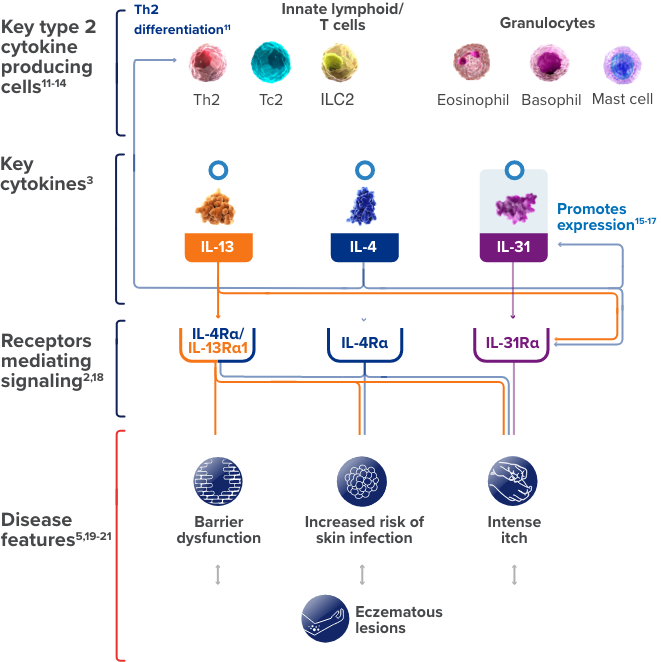
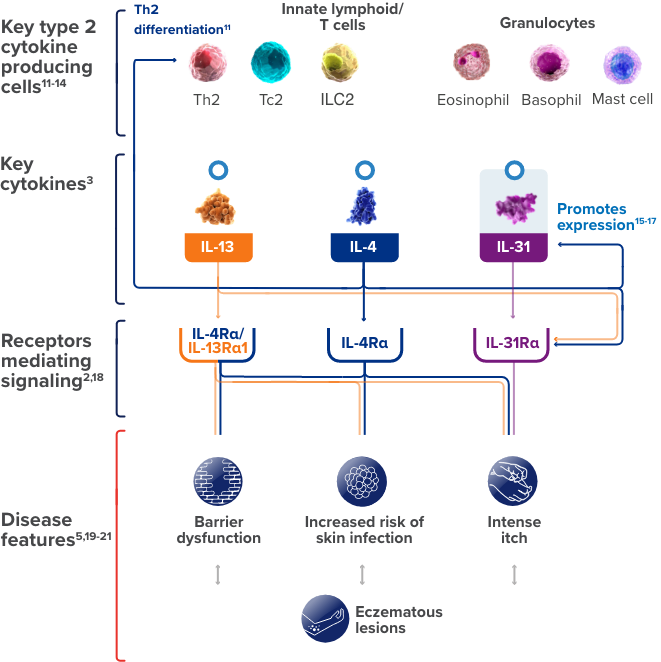
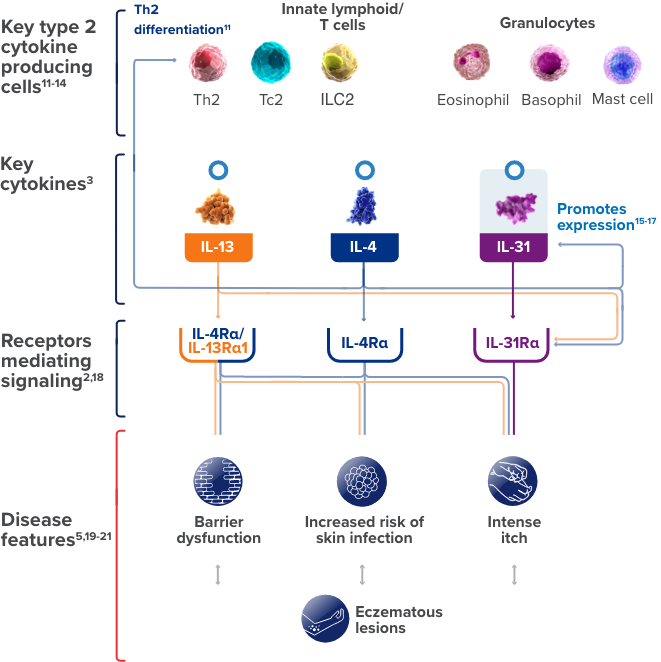
This is a simplified illustration of our current understanding about the possible roles of IL-4, IL-13, and IL-31,
three of the cytokines involved in type 2 inflammation, in contributing to certain
features of atopic dermatitis.
ILC, innate lymphoid cell; Tc, cytotoxic T cell.
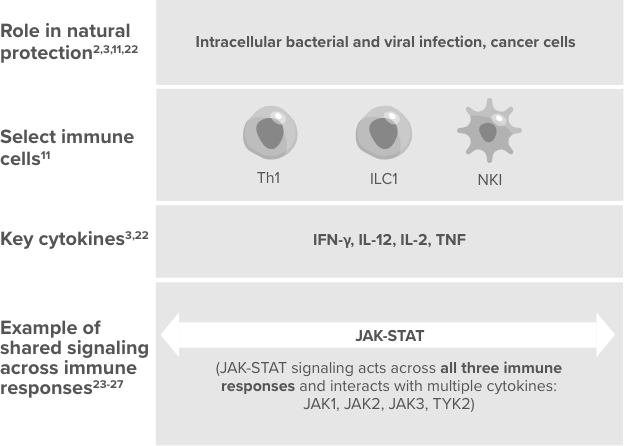

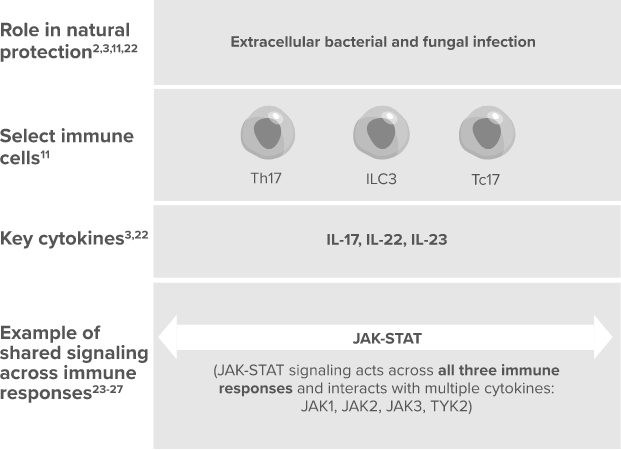
While the 3 types of immune responses are well recognized, the above illustration is a simplification. The 3 immune responses may functionally overlap, interact, and change over time.
When dysregulated, immune
responses can contribute to disease11
IFN-γ, interferon gamma; JAK, janus kinase; NFAT, nuclear factor of activated T-cell; NK, natural killer cell; STAT, signal transducer of activation; TNF, tumor necrosis factor; TYK2, tyrosine kinase 2.
References: 1. Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124(1):28-35. 2. Gandhi NA, Bennett BL, Graham NMH, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35-50. 3. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. 4. Hamilton JD, Ungar B, Guttman-Yassky E. Drug evaluation review: dupilumab in atopic dermatitis. Immunotherapy. 2015;7(10):1043-1058. 5. Rerknimitr P, Otsuka A, Nakashima C, Kabashima K. The etiopathogenesis of atopic dermatitis: barrier disruption, immunological derangement, and pruritus. Inflamm Regen. 2017;37:14. 6. Brunner PM, Suárez-Fariñas M, He H, et al. The atopic dermatitis blood signature is characterized by increases in inflammatory and cardiovascular risk proteins. Sci Rep. 2017;7(1):8707 [published correction appears in Sci Rep. 2018;8(1):8439]. 7. Palomares O, Akdis M, Martín-Fontecha M, Akdis CA. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunol Rev. 2017;278(1):219-236. 8. Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133(2):448-460. 9. Murota H, Katayama I. Exacerbating factors of itch in atopic dermatitis. Allergol Int. 2017;66(1):8-13. 10. Suárez-Fariñas M, Tintle SJ, Shemer A, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127(4):954-964. 11. Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135(3):626-635. 12. Park K, Park JH, Yang WJ, Lee JJ, Song MJ, Kim HP. Transcriptional activation of the IL31 gene by NFAT and STAT6. J Leukoc Biol. 2012;91(2):245-257. 13. Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13(8):607-614. 14. Hashimoto T, Kursewicz CD, Fayne RA, et al. Pathophysiologic mechanisms of itch in bullous pemphigoid. J Am Acad Dermatol. 2020;83(1):53-62. 15. Stott B, Lavender P, Lehmann S, Pennino D, Durham S, Schmidt-Weber CB. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J Allergy Clin Immunol. 2013;132(2):446-454. 16. Miake S, Tsuji G, Takemura M, et al. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced Ccl 17 and Ccl 22 production in dendritic cells: implications for atopic dermatitis. Int J Mol Sci. 2019;20(16):4053. 17. Edukulla R, Singh B, Jegga AG, Sontake V, Dillon SR, Madala SK. Th2 cytokines augment IL-31/IL-31RA interactions via STAT6-dependent IL-31RA expression. J Biol Chem. 2015;290(21):13510-13520. 18. Rabenhorst A, Hartmann K. Interleukin-31: a novel diagnostic marker of allergic diseases. Curr Allergy Asthma Rep. 2014;14(4):423. 19. Silverberg JI, Kantor R. The role of interleukins 4 and/or 13 in the pathophysiology and treatment of atopic dermatitis. Dermatol Clin. 2017;35(3):327-334. 20. Erickson S, Nahmias Z, Rosman IS, Kim BS. Immunomodulating agents as antipruritics. Dermatol Clin. 2018;36(3):325-334. 21. Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43(1):29-40. 22. Kaiko GE, Horvat JC, Beagley KW, Hansbro PM. Immunological decision-making: how does the immune system decide to mount a helper T-cell response? Immunology. 2008;123(3):326-338. 23. Morris R, Kershaw NJ, Babon JJ. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018;27(12):1984-2009. 24. Yoshida H, Nishina H, Takimoto H, et al. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8(1):115-124. 25. Park YJ, Yoo SA, Kim M, Kim WU. The role of calcium-calcineurin-NFAT signaling pathway in health and autoimmune diseases. Front Immunol. 2020;11:195. 26. A Reference Guide for Jak/STAT Signaling: R&D Systems. Accessed July 28, 2021. https://www.rndsystems.com/resources/articles/reference-guide-jak-stat-signaling 27. Schindler C, Plumlee C. Interferons pen the JAK-STAT pathway. Semin Cell Dev Biol. 2008;19(4):311-318.